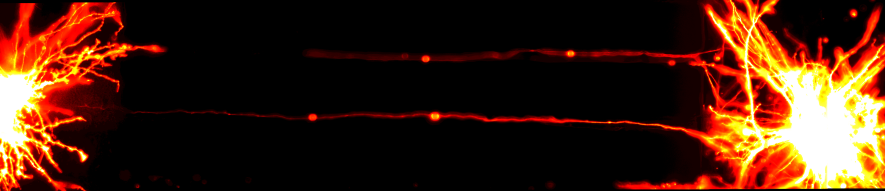
 Shining Light on Neurodegeneration
Shining Light on Neurodegeneration
Research summary
The Molecular Neuroscience Group focuses on investigating the molecular mechanisms that can cause neurodegenerative diseases, such as Parkinson’s disease (PD), Alzheimer's disease (AD), and Huntington’s disease (HD).
For more information, visit the group website at: https://www.ceb-mng.org/

