In this section we outline the key principles and processes occurring within a range of different fuel cells.
Background
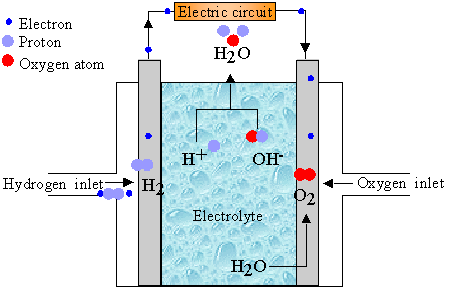
Electricity is perhaps the most versatile and easy to exploit form of energy. However its generation currently requires either large scale gas/oil/coal based power stations or the use of alternative sustainable approaches such as wind, hydroelectric or solar cells. Fuel cell technology offers the opportunity of creating environmentally friendly portable power supplies capable of producing enough energy to run devices and motor vehicles. The basic principle is simple: Oxygen from the air is reacted with hydrogen (Stored in the device) to form water. This is a redox process in which electrons are transferred, the trick of a fuel cell is to use the electrons generated during the reaction to perform work. A schematic of the basic fuel cell components is shown below
Historical Perspective
The fuel cell is not a recent invention. Sir Humphrey Davy created in 1802 a simple fuel cell (C | H2O, HNO3| O2 | C) with which he could give himself a feeble electric shock. In 1839 Sir William Grove (who is often referred to as the 'Father of the fuel cell') created the first cell type base on reversing the electrolysis of water. He argued that by reversing this process electricity could be generated by the reaction of hydrogen and oxygen together. Though the first fuel cell is nearly two centuries old, its development towards real world applications has been relatively slow. This is in part is due to the 'success' of the internal combustion engine which although inefficient, produces more than enough power for general applications in a range of industries. Another aspect holding back the successful application of fuel cells has also been the relatively complex materials science/chemistry which has to be optimised.
The term 'fuel cell' was first used by Ludwig Mond and Charles Langer in 1889, and this was followed by extensive work to try and develop usable devices. 1932 saw the first successful application of a fuel cell and this was demonstrated by Francis Bacon, By 1959 Bacon and coworkers demonstrated a 5KW system which was capable of powering welding machinery. Also around this period Harry Karl Ihrig demonstrated a 20 horse power tractor driven by Fuel cells. The 1960's saw a rapid expansion of research within the area, partly driven by funding from NASA who were looking for low weight, clean and highly efficient electricity sources for their space programme. An added bonus of the hydrogen fuel cell is the production of water (and heat) which was also extremely useful within their space craft.
In the period 1970-1990, Japanese companies also became a big investors in fuel cell research. By the 1980s Japan had small scale power stations operating and extensive interest from the automotive industry. For further details and links to a range of companies currently involved in fuel cell technology see: Construction and Applications of fuel cells.
Operating Principles
Fuel cells are often referred to as continuously operating batteries. They exploit electrolysis reactions in a similar manner to traditional batteries however the reagents are constantly resupplied to the cell and hence they do not become discharged like traditional batteries. An animation of the basic operation of a hydrogen fuel cell can be found at www.ballard.com.
In this case the two fuels are oxygen and hydrogen producing water (and heat) as the products. The hydrogen is fed to the 'fuel electrode' where it is oxidised into H+ ions. The oxygen is fed to the 'air electrode' where it is reduced to OH- ions through reaction with water in the electrolyte. Both those ions meet in the electrolyte between the two electrodes to form water. During this process, the electrons taken from the hydrogen are taken into a external electric circuit before returning to the cathode to form the OH- ions.

The thermodynamics of the reaction limits the theoretical voltage that can be generated from a single pair of electrodes to about 1.2V. If a more substantial voltage is required then a number of these cells are 'stacked'. We will now look in more detail at the key cell components.
The Fuel
Oxygen is usually taken from the air (filtered an dcleaned before introduction to the cell) and hydrogen is generally made from the decomposition of a hydrocarbon soucre (such as methane). For further details see the fuel.
The Electrodes
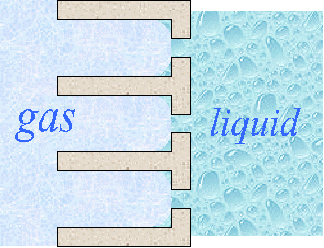
The function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself being consumed or corroded. They must also, by definition, be an electronic conductor and bring the three phases (gaseous fuel, liquid electrolyte and electrode itself) to contact. There are several methods for gas-liquid interface stabilisation which , rely on capillary action. A biporous electrode allows liquid to penetrate the small pores and a gas pressure is applied that drives the liquid out of the large pores. All this is set-up within the structure of the electrode. Then the merging of the two phases may be achieved in several ways. The electrolyte will tend to form a thin wetting film over part of the internal surface of the electrode. The reactant gas, sparingly soluble in the electrolyte, can diffuse through this film and reach the electrode surface, where a solid-liquid reaction can occure. The electrode structure have to be designed to maximised the area of the wetted film.
The Electrolyte
A large range of fuel cells have been developed using a variety of difference electolytes. These developments have been driven by the desire to improve the economics of the cells and the potential applications at a rang eof different temperatures. In fact fuel cells are usually classified in terms of their electolyte contents. For a detailed overview of the differing cell types (eg alkaline, Sulfuric Acid, Phosphoric acid, Proton Exchange Membrane, Solid Polymer, Molten Carbonate & Solid Oxide) see fuel cells

