Electron transfer plays a fundamental role in governing the pathway of chemical reactions. Yet the speed and size of the electron mean that tracing its movement is difficult using tradition methods such as spectroscopy and synthetic chemistry. Consequently our knowledge of the driving force for many reactions remains elusive. Electrochemical methods offer the potential to investigate these processes directly by the detection of the electrons involved. This course will highlight the main principles behind the kinetic aspects of electrochemistry and concentrate on the application of electrochemical techniques to the investigation of charge transfer driven processes. In the following sections a general overview of electrolysis reactions is covered.
Electrode Reactions
A typical electrode reaction involves the transfer of charge between an electrode and a species in solution. The electrode reaction usually referred to as electrolysis, typically involves a series of steps:
- Reactant (O) moves to the interface: this is termed mass transport
- Electron transfer can then occur via quantum mechanical tunnelling between the electrode and reactant close to the electrode (typical tunnelling distances are less than 2 nm)
- The product (R) moves away from the electrode to allow fresh reactant to the surface
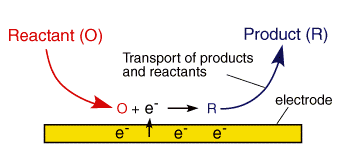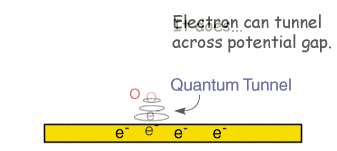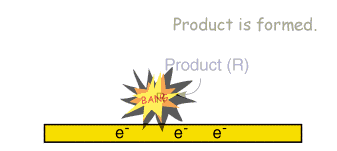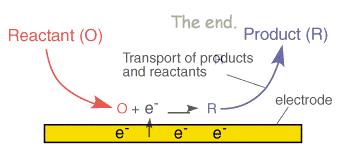
The above electrode steps can also be complicated by:
-
The applied voltage on the electrode
-
The reactivity of the species
-
The nature of the electrode surface
-
The structure of the interfacial region over which the electron transfer occurs
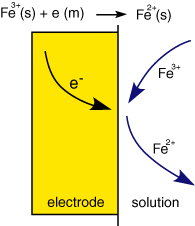
Before we begin to look at the above processes in detail, let us consider a few examples of electrode reactions. Perhaps the 'simplest' is a single electron transfer, a schematic of which is shown to the right: Here the reactant Fe3+ moves to the interface where it undergoes a one electron reduction to form Fe2+. The electron is supplied via the electrode which is part of a more elaborate electrical circuit. For every Fe3+ reduced a single electron must flow. By keeping track of the number of electrons flowing (ie the current) it is possible to say exactly how many Fe3+ molecules have been reduced.
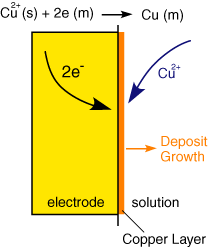
A further example of electrochemistry in action is metal deposition, shown in the figure on the left: In this case the electrode reaction results in the fomation of a thin film on the orginal surface. It is possible to build up multiple layers of thin metal films simply by passing current through appropriate reactant solutions.
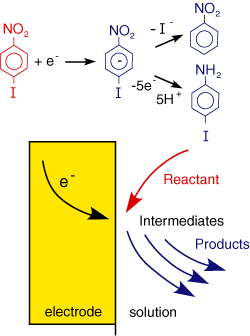
A final example of electrode reactions is shown in the figure on the right: In this case an organic molecule is reduced at the electrode forming the radical anion. This species however is unstable and undergoes further electrode and chemical reactions. We will see later how electrochemistry can be employed to study and initiate a whole range of chemical processes.
Equilibrium Electrochemistry
The above are all examples of electrolysis reactions, where an electron is forced in or out of the electrode. In this situation a current flows for as long as the reaction continues. This differs significantly from the electrochemical topics you have encountered in earlier courses. Before we begin to look at electrolysis in detail it is perhaps useful to briefly recall some points from the year I course on equilibrium electrochemistry. Then typically we were using a two compartment cell with separate reactants in each side. When electrodes were placed within each compartment and a circuit made between the two halves, a voltage was obtained. It is important to note that this measurement is performed so that no current is allowed to flow between the compartments. Hence we obtain an equilbrium. The voltage measured then predicts which way electrons would like to flow (if they could), this is purely thermodynamic measurement. Like data from chemical equilibrium measurements the infomation tells us whether it is thermodynamically favourable or not for a reaction to proceed. Such experiments allow the measurement of the following quantities:
- Enthalpies of reaction
- Entropies of reaction
- Free energies of reaction
- Equilibrium constants
- Solution pH
However these measurements reveal nothing regarding the kinetics of the process. To gain kinetic information we must watch the establishment of the equilibrium - this is essentially the area of electrode dynamics, the topic of the lecture course. We will examine a model of the kinetic in the next document, before we do this however, we need to gain a microscopic view of why electrode reactions occurr, and what this odd term voltage has to do with 'chemistry'.
Electron Transfer and Energy Levels
The key to driving an electrode reaction is the application of a voltage (V). If we consider the units of volts:
V = Joule/Coulomb
we can see that a volt is simply the energy (J) required to move charge (c). Application of a voltage to an electrode therefore supplies electrical energy. Since electrons possess charge an applied voltage can alter the 'energy' of the electrons within a metal electrode. The behaviour of electrons in a metal can be partly understood by considering the Fermi-level (EF). Metals are comprised of closely packed atoms which have strong overlap between one another. A piece of metal therefore does not possess individual well defined electron energy levels that would be found in a single atom of the same material. Instead a continum of levels are created with the available electrons filling the states from the bottom upwards. The Fermi-level corresponds to the energy at which the 'top' electrons sit.
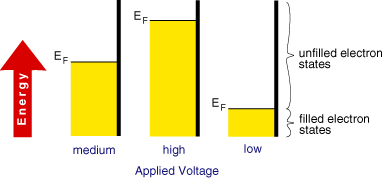
This level is not fixed and can be moved by supplying electrical energy. Electrochemists are therefore able to alter the energy of the Fermi-level by applying a voltage to an electrode.
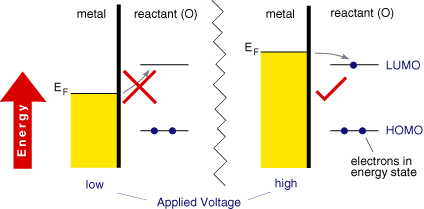
The figure below shows the Fermi-level within a metal along with the orbital energies (HOMO and LUMO) of a molecule (O) in solution. On the left hand side the Fermi-level has a lower value than the LUMO of (O). It is therefore thermodynamically unfavourable for an electron to jump from the electrode to the molecule. However on the right hand side, the Fermi-level is above the LUMO of (O), now it is thermodynamically favourable for the electron transfer to occur, ie the reduction of O.
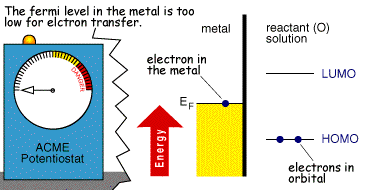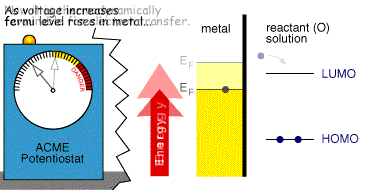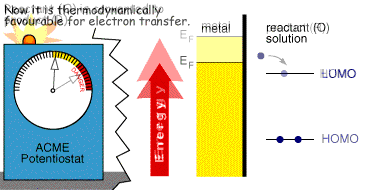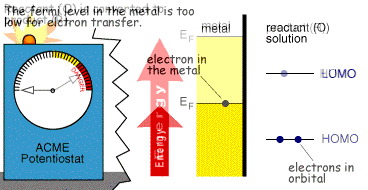
Whether the process occurs depends upon the rate (kinetics) of the electron transfer reaction and the next document describes a model which explains this behaviour

