Voltammetry is one of the techniques which electrochemists employ to investigate electrolysis mechanisms. There are numerous forms of voltammetry:
-
Potential step
-
Linear sweep
-
Cyclic voltammetry
For each of these cases a voltage or series of voltages are applied to the electrode and the corresponding current that flows monitored. In this section we will examine potential step voltammetry, the other forms are described on separate pages
Experimental Cell
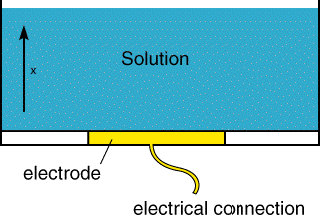
For the moment we will focus on voltammetry in stagnant solution. The figure below shows a schematic of an electrolysis cell. There is a working electrode which is hooked up to an external electrical circult. For our purposes at the moment we will not worry about the remainder of the circuit, obviously there must be more than one electrode for current to flow. But as we shall see later it is only the so called working electrode that controls the flow of current flow in the electrochemical measurement.
As the electrolysis continues material can diffuse further from the electrode and therefore the concentration gradient drops. As the concentration gradient drops (see concentration profiles below) so does the supply of fresh reactant to the surface and therefore the current also decreases.
The essential elements needed for an electolysis measurement are as follows:
- The electrode: This is usually made of an inert metal (such as Gold or Platinum)
- The solvent: This usually has a high dielectric constant (eg water or acetonitrile) to enable the electrolyte to dissolve and help aid the passage of current.
- A background electrolyte: This is an electrochemically inert salt (eg NaCl or Tetra butylammonium perchlorate, TBAP) and is usually added in high concentration (0.1M) to allow the current to pass.
- The reactant: Typically in low concentration 10-3 M
Potential Step Voltammetry
In the potential step measurement the applied voltage is instantaneously jumped from one value V1 to another V2
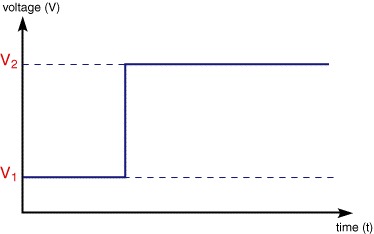
The resulting current is then measured as a function time. If we consider the reaction:
![]()
Usually the voltage range is set such that at V1 the reduction of Fe3+ is thermodynamically unfavourable. The second value of voltage (V2) is selected so that any Fe3+ close to the electrode surface is converted to product ,Fe2+. Under these conditions the current response is shown below
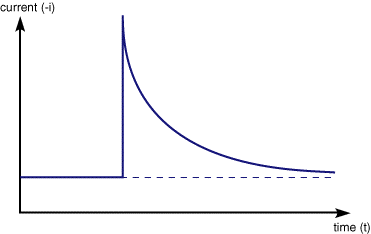
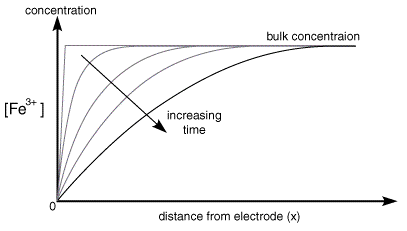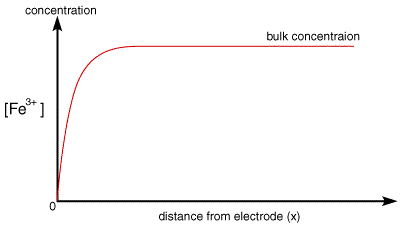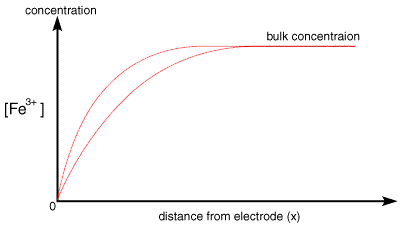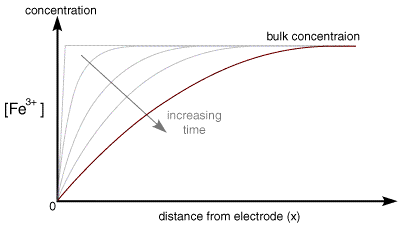
The current rises instantaneously after the change in voltage and then begins to drop as a function of time. This occurs since the instant before the voltage step the surface of the electrode is completely covered in the reactant and the solution has a constant composition below

Once the step occurs reactant (Fe3+) is converted to product (Fe2+) and a large current begins to flow. However now for the reaction to continue we need a supply of fresh reactant to approach the electrode surface. This happens in stagnant solution via diffusion. As we noted in a previous section the rate of diffusion is controlled by the concentration gradient. So the supply of fresh (Fe3+) to the surface (and therefore the current flowing) depends upon the diffusional flux. At short times the diffusional flux of (Fe3+) is high, as the change in concentration between the bulk value and that at the surface occurs over a short distance
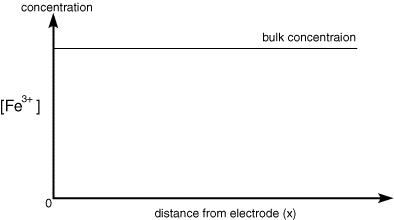
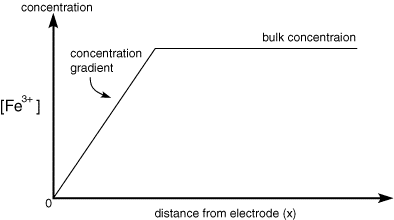
As the electrolysis continues material can diffuse further from the electrode and therefore the concentration gradient drops. As the concentration gradient drops (see concentration profiles below) so does the supply of fresh reactant to the surface and therefore the current also decreases.
For a large electrode the appropriate mass transport equation for the reaction is
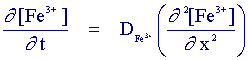
where the distance x is normal to the electrode surface. If we recall the expression for the electrolysis current for a reduction reaction:
![]()
Clearly this is not particularly helpful for experimental purposes since we require the surface concentration as a function of time. However Cottrell showed it is possible to rearrange and solve the mass transport equation in terms of the flux. This gives
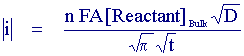
Now the current is related to the bulk reactant concentration and we can see by inspection that if the current is measured as a function of time we would expect a plot of
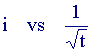
to be a straight line if the reaction is occuring by diffusion control. Such plots allow the estimation of the diffusion coefficients of the species to be obtained.

