The study of processes occuring at the immiscible liquid/liquid interface have been advanced considerably by the implementation of a microfluidic approach. This enables the creation of a stable interface for confluent immiscible streams or the formation of monodisperse droplets using a flow focusing approach. The group has used microfluidic techniques to study the kinetics and mechanisms of processes occurring at the immiscible liquid/liquid interface such as two-phase chemical reactions, phase transfer catalysis, ion extraction, nanoparticle formation and photoelectrochemical reactions. Some of these studies are described below.
Ion transfer at the immiscible liquid/liquid interface
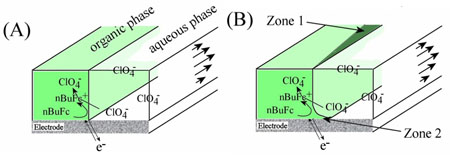
One of our recent projects in collaboration with Dr Frank Marken (University of Bath) has been to elucidate the mechanism of ion transfer at the dynamic immiscible liquid/liquid interface which can be an important component of electrochemical synthesis reactions. The study focused on the oxidation of n-butylferrocene at the organic/aqueous/electrode triple phase boundary: ![]()
As described in the voltammetry section, the Levich equation predicts that the limiting current produced by a channel electrode increases with volume flow rate. However, in this case the opposite is observed. This can be explained by considering the reaction zone which grows towards the trailing edge of the electrode (zone 1 in the schematic below). At faster flow rates this reaction zone is diminished and the current is reduced. The currents measured correspond to conversion rates of approximately 0.01%; this can be improved to about 10% by lengthening the working electrode and lowering the volume flow rate.
Droplet based microfluidic systems
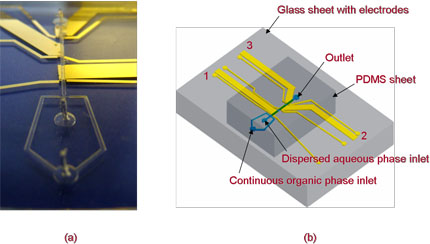
Some studies require the use of a discontinuous immiscible liquid/liquid interface; one way of generating this is to use a droplet-based microfluidic system. The use of a microfluidic approach ensures highly monodisperse droplet production and pico/femtolitre droplet volumes; this small scale ensures a high surface area to volume ratio and enhances heat and mass transfer. Examples of experimental systems studied include: the quantification of enzyme kinetics, DNA amplification, protein expression and crystalisation studies. A key challenge in the development of the droplet-based microfluidic devices is the characterisation of key parameters such as droplet size, frequency and composition. A popular approach has been the use of optical techniques; however, whilst very succuesful this does require the use of buly and expensive equipment as well as optically transparent devices. In collaboration with Prof. Wilhelm Huck (Department of Chemistry) we have developed a novel electrochemical approach for the characterisation of droplet-based microfluidic devices. The device developed is based on a typical flow-focusing geometry to generate droplets and incorporates 3 sets of reference, working and counter electrodes as shown in the photo and schematic below. In these electrochemical experiments the continuous organic stream contains an electroactive species such as TMPD and a background electrolyte such as TBAP.
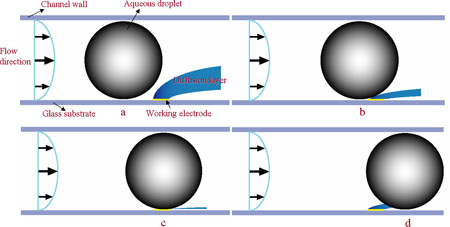
Once a steady stream of droplets has been formed a potential step experiment is performed; under normal flow conditions (with no droplets) a stedy state current, ilim, is observed. When droplets are flowing through the device a pertubation from the steady state current is observed, as shown in the figure below.
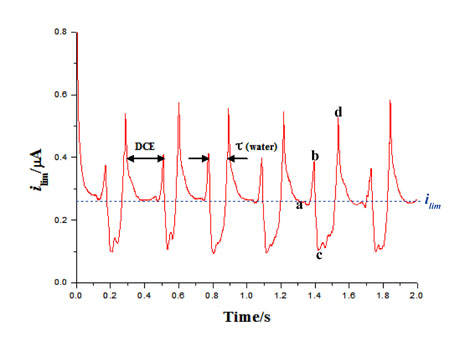
We believe that the pertubation from the steady state is due to the droplet interferring with the growth of the diffusion layer above the electrode. This is shown in the diagram below. The diagrams a,b,c and d correspond to the points marked on the graph above