Imaging and Spectroscopic Methods in Microfluidic Devices
A number of the group's members use a variety of imaging techniques in order to provide an insight into physical processes occurring within the microfluidic devices. Using these techniques we have studied processes such as:
- Mass transport
- Chemical reaction kinetics
- Electrolysis reactions
- Immiscible liquid/liquid flow
- Velocity profiles in both miscible and immiscible systems
- Electron transfer and mass transport in biological systems
This information can be obtained using analytical techniques such as Confocal Microscopy, Fluorescence Lifetime Imaging Microscopy (FLIM), Magnetic Resonance Imaging (MRI) and Electron Spin Resonance Spectroscopy (ESR).
Quantitative information can also be obtained by combining the experimental technique with numerical simulations.
Confocal Microscopy
Using the department's SRIF funded confocal microscope facility we have quantitatively analysed diffusive mixing within microfluidic devices for both chemical and biochemical systems. T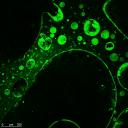 his technique allows high resolution xyz imaging of the devices as well as the imaging of time evolutionary processses. The figure to the right shows the imaging of a fluorescent protein (LacI-GFP) immobilised on porous poly(HEMA-co-EGDMA-co-AGEIDA complex). Further developments include the imaging of electron transfer and subsequent mass transport processes within biological systems to aid the understanding and optimisation of bio-photovoltaic devices. An image of the excited photosynthetic apparatus of the algae Chlorela is shown below. his technique allows high resolution xyz imaging of the devices as well as the imaging of time evolutionary processses. The figure to the right shows the imaging of a fluorescent protein (LacI-GFP) immobilised on porous poly(HEMA-co-EGDMA-co-AGEIDA complex). Further developments include the imaging of electron transfer and subsequent mass transport processes within biological systems to aid the understanding and optimisation of bio-photovoltaic devices. An image of the excited photosynthetic apparatus of the algae Chlorela is shown below. |
|
 |
Fluorescence Lifetime Imaging Microscopy (FLIM)
|
|
|
|
|
Magnetic Resonance Imaging (MRI)
| In collaboration with the Magnetic Resonance Research Centre (MRRC) in the department, we have imaged microfluidic devices using MRI. This technique enables the acquisition of either concentration or velocity profiles. The image to the right is a velocity profile of single phase fluid flow within a rectangular microchannel, clearly illustrating stable laminar flow. One of the key benefits of the MRI technique is the ability to image confluent immisicible liquids flowing through a channel. This can be used to provide information about the position and shape of the immisicible liquid interface as well as velocity profiles of the two streams. The image on the right is an example of an intensity based image of two immisicible liquids flowing in a microchannel; the position and shape of the interface are dependent on a number of factors such as the relative viscosities and the relative flow rates of the two liquids. The image below is a velocity image of an immisicible system; this provides valuable information about the fluid flow within these systems. | 
|
 |
Electron Spin Resonance Spectroscopy (ESR)
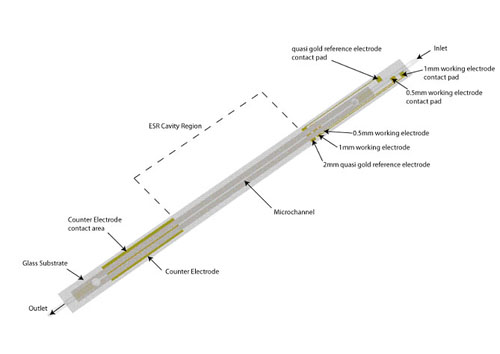
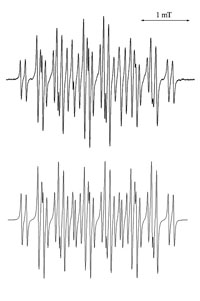 Traditionally, the application of ESR techniques to identify radical species produced by electrochemical methods have been succesful, but ultimately limited by the sensitivity of the spectroscopic method. By using microfluidic devices it is possible to improve the signal to noise ratio by decreasing the dielectric losses of the system. In collaboration with Prof. Richard Compton at the University of Oxford we have developed devices to be used both at room temperature and -40C, a schematic of the device is shown below. The upper image to the left is an experimental ESR spectrum of the electrochemically generated m-iodobenzene radical anion at -40C, the image below is the simulated spectrum.
Traditionally, the application of ESR techniques to identify radical species produced by electrochemical methods have been succesful, but ultimately limited by the sensitivity of the spectroscopic method. By using microfluidic devices it is possible to improve the signal to noise ratio by decreasing the dielectric losses of the system. In collaboration with Prof. Richard Compton at the University of Oxford we have developed devices to be used both at room temperature and -40C, a schematic of the device is shown below. The upper image to the left is an experimental ESR spectrum of the electrochemically generated m-iodobenzene radical anion at -40C, the image below is the simulated spectrum.


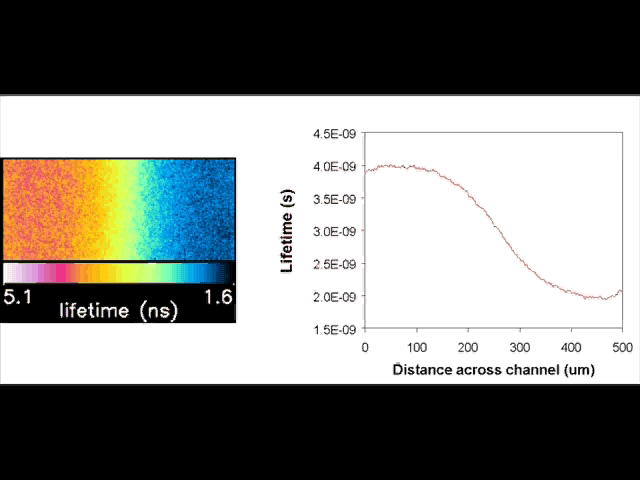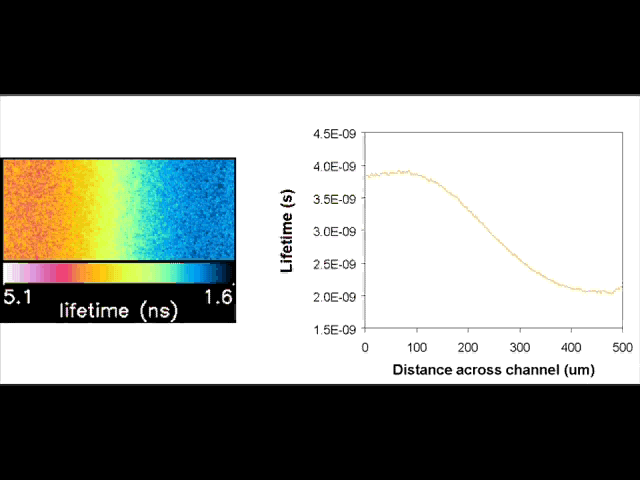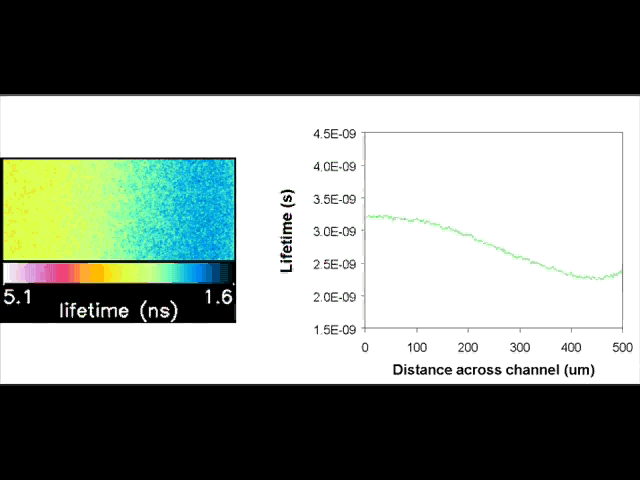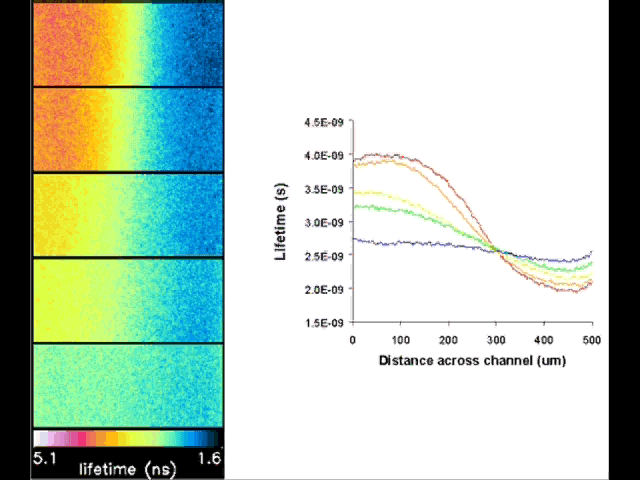
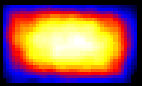
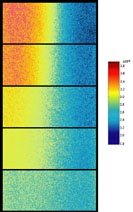 Fluorescent lifetime imaging microscopy (FLIM) is a powerful alternative to traditional, intensity based, fluorescence measurements. In this technique it is the fluorescent lifetime that is used to differentiate between species; providing a method that is able to quantify processes such as pH variations, diffusional mobility, conformational changes and quenching. We have worked in collaboration with the Laser Analytics Group in the department to develop the use of FLIM for quantitatively studying mass transport and reaction kinetics within microfluidic devices. The image to the left shows the fluorescent lifetime images for a dual-inlet microfluidic device where both inlets contain a fluorophore (Rhodamine 6G); one inlet also contains potassium iodide, which quenches the Rhodamine 6G fluorescence. The lifetime images obtained at increasing distances downstream illustrate the diffusion of the iodide ions into the other stream resulting in a uniform fluorescent lifetime across the width of the channel. The animation below shows the fluorescent lifetime images and profiles across the width of the channel at increasing distances downstream.
Fluorescent lifetime imaging microscopy (FLIM) is a powerful alternative to traditional, intensity based, fluorescence measurements. In this technique it is the fluorescent lifetime that is used to differentiate between species; providing a method that is able to quantify processes such as pH variations, diffusional mobility, conformational changes and quenching. We have worked in collaboration with the Laser Analytics Group in the department to develop the use of FLIM for quantitatively studying mass transport and reaction kinetics within microfluidic devices. The image to the left shows the fluorescent lifetime images for a dual-inlet microfluidic device where both inlets contain a fluorophore (Rhodamine 6G); one inlet also contains potassium iodide, which quenches the Rhodamine 6G fluorescence. The lifetime images obtained at increasing distances downstream illustrate the diffusion of the iodide ions into the other stream resulting in a uniform fluorescent lifetime across the width of the channel. The animation below shows the fluorescent lifetime images and profiles across the width of the channel at increasing distances downstream.