Submitted by Ellie Hall on Fri, 24/03/2023 - 14:11
Researchers from the Department of Chemical Engineering and Biotechnology and the Cambridge Centre for Advanced Research and Education in Singapore (CARES) have made a significant step towards understanding the mechanism for electrocatalytic CO2 reduction.
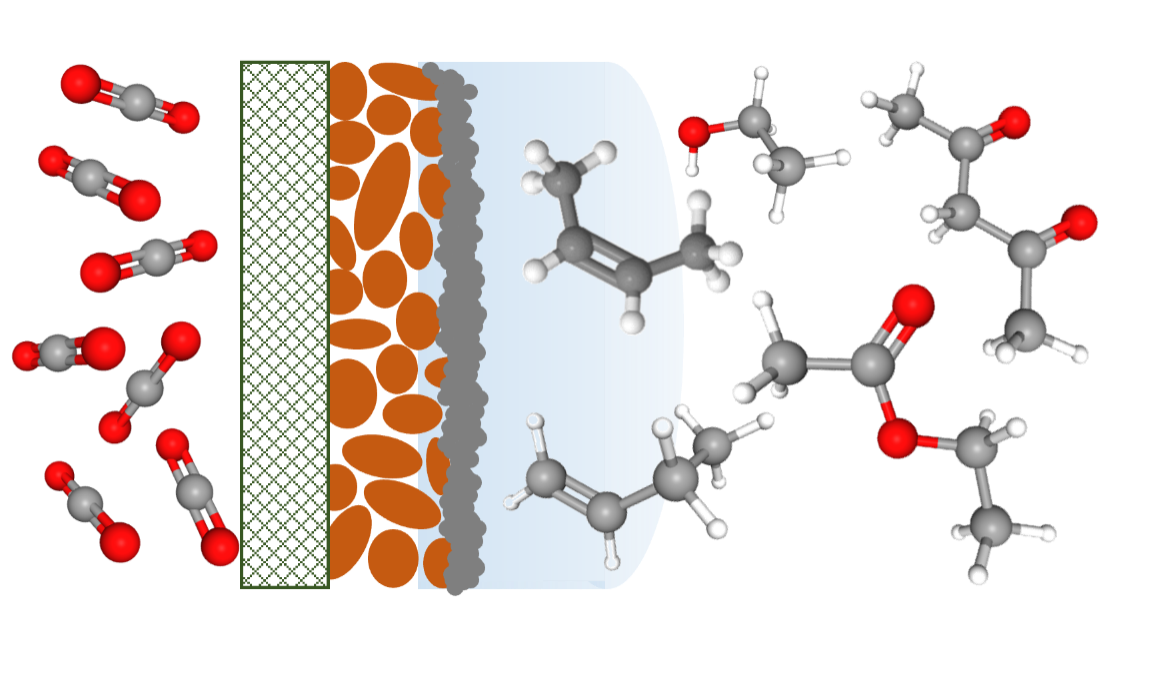
Electrocatalytic reduction of carbon dioxide (CO2) is a process of passing an electrical current through a metal catalyst in a solution of CO2, causing the CO2 molecules to react with water, forming products such as methane, ethylene, or ethanol. If scaled up, this process could sustainably and efficiently reduce CO2 emissions by converting them into useful chemicals, which can be used as fuels or feedstocks for the chemical industry. However, process development under commercially-relevant conditions is still in its early stages and faces challenges such as low selectivity, instability, and catalyst deactivation due to contamination.
“Understanding this reaction mechanism is crucial for two reasons: first, it reveals what products are possible and whether they can be scaled up, and second, it shows what reaction paths are responsible for these products so we can engineer systems to favour or disfavour them,” said Simon Rihm, the study’s co-first author.
The study, published in Energy & Environmental Science, focuses on the electrochemical reduction of CO2 to various hydrocarbons using copper-based electrodes. Copper-based electrodes are particularly promising because they can produce many valuable products, such as ethylene. The team used ultra-sensitive analytical techniques to identify and examine ten previously unknown minor products of electrochemical CO2 reduction, including large 5-carbon-chain molecules, for the first time.
After thorough investigations, the researchers discovered that there are two main reaction paths active at significant current densities: one that favours double bond formation in the middle of carbon chains, and another that leads to double bond formation at the chain end. This breakthrough in understanding will help researchers design better catalysts with improved selectivity and reduced contamination, which is crucial for developing more efficient CO2 utilisation technologies.
“Further study into reaction paths may allow us to enhance the efficiency of CO2 utilisation and ultimately provide a viable negative emissions technology, which will be critical for achieving climate mitigation targets,” said Markus Kraft, the corresponding author of the study, and head of Cambridge’s Computational Modelling Group.
Image caption: Diagram showing carbon dioxide molecules passing through an electrocatalyst, which converts them to higher value molecules such as ethylene.
Image credit: Simon Rihm