Submitted by Administrator on Fri, 12/04/2019 - 16:11
Research from our Cardoso lab and their colleagues in Spain, published in Angewandte Chemie, combines two classic demos in one beautiful example of active fluid dynamics.
Chemical gardens and colour-changing clock reactions are two classic chemistry demonstrations that have been inspiring budding scientists for decades.
Chemical gardens are usually made by adding metal salts, such as copper sulfate or cobalt(II) chloride, to an aqueous solution of sodium silicate, otherwise known as waterglass. When a metal salt is added to a sodium silicate solution it starts to dissolve, forming an insoluble metal silicate, which has a semipermeable membrane. Because the ionic strength of the metal solution inside the membrane is higher than the sodium silicate solution surrounding the membrane, water begins to move into the membrane, increasing the pressure. This causes the membrane to tear, forming a hole at which the metal ions can react with the silicate ions to form a new solid. In this way, plant-like growths will form in the tanks, coloured according to the metals used.
While chemical gardens provide fun, attractive demos, they have also been shown to have real-world applications, explaining reactions taking place in corrosion, cement hydration and the origin of life at hydrothermal vents at the bottom of the ocean.
 A chemical clock is a mixture of reacting chemical compounds in which a visible change in properties of the mixture occurs after a specific time has passed. This is often a colour change: if one of the chemicals has a visible colour, crossing a particular concentration threshold can lead to an abrupt colour change at a specific and reproducible time.
A chemical clock is a mixture of reacting chemical compounds in which a visible change in properties of the mixture occurs after a specific time has passed. This is often a colour change: if one of the chemicals has a visible colour, crossing a particular concentration threshold can lead to an abrupt colour change at a specific and reproducible time.
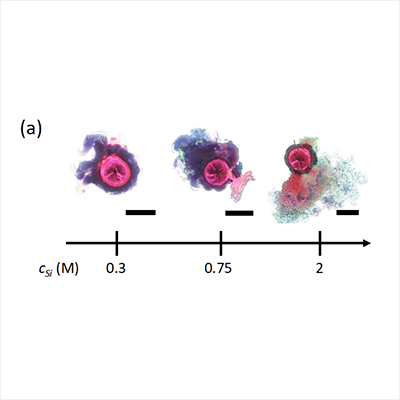
Until now chemical gardens and chemical clocks have been considered entirely separate categories of reaction. But research by Yang Ding, a PhD student in Professor Silvana Cardoso’s group, has shown that a chemical garden constrained to grow in two dimensions will show a controlled explosion of the garden at a consistent and reproducible time: a chemical garden that is also a chemical clock.
Their chemical garden is made by adding cobalt chloride to sodium silicate. The cobalt silicate membrane forms, surrounding the cobalt chloride. This membrane is initially impermeable to both the cobalt and silicate ions, but permeable to water, so that an osmotic pressure gradient develops across it. This drives water from the exterior environment into the cell enclosed by the membrane, increasing the internal pressure.
This increase in pressure opens up small cracks in the membrane and pumps the solution of cobalt chloride outwards through these. A dual-permeability membrane therefore develops: the chemistry controls the low permeability of ions moving into the membrane, while the internal pressure and solid mechanics of the membrane control the higher permeability of ions moving out of the membrane. In all experiments, the outward flow of ions decreases with time and the chemical garden ultimately explodes.
The timing of the explosion can be carefully controlled by altering the concentrations of the solutions – creating a chemical garden that acts as a timer.
“When I was a child, I observed osmotic explosions of red blood cells in biology classes," said Yang Ding. "I also grew chemical gardens in chemistry classes. People didn’t know that there is actually a link between osmotic explosions and chemical gardens. It is the first observation of such beautiful physical explosions in the inorganic world. We are currently in collaboration with our colleagues in Granada, Spain to explore the osmotic explosions within macro-biological field.”
Chemical‐garden reactions in general have not previously been shown to have clock reaction dynamics. This research could help us to better understand the interaction of chemistry and fluid mechanics at hydrothermal vents, which host rich and diverse minerals and life.
Read the full paper, published in Angewandte Chemie, here and watch a video of the explosion below.
Video field view 12.5 x 12.5 mm2. The group would like to thank Dr Simon Butler for his help in filming this video.

