Submitted by Ellie Hall on Thu, 18/07/2019 - 14:52
“Without you, we would not have gotten to the moon”.
So said President Richard Nixon to Francis Thomas (Tom) Bacon, referring to Bacon’s invention of the first practical hydrogen-oxygen fuel cell, named one of Britain’s greatest innovations by the Foreign and Commonwealth Office in 2017.
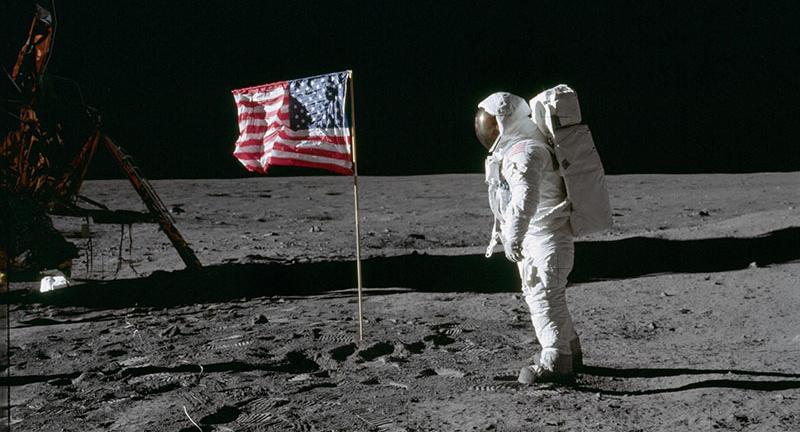
Saturday 20 July 2019 marks the 50th anniversary of the moon landings, and while most of us are very familiar with the iconic scenes of this ‘giant leap for mankind’, many of us are completely unaware of the dinner plate-sized invention that made Neil Armstrong’s ‘small step’ possible.
Tom Bacon was working in our department back in the 1950's when he made the breakthrough that led to the development of the highly efficient fuel cells used to power the Apollo 11 spacecraft.
Sir William Grove had demonstrated the concept of a fuel cell – a device that produces electricity directly from a chemical reaction – in 1839, but Bacon was able to take his initial investigations further, using his experience as an engineer. Comfortable working with machinery operating at high temperatures and pressures, Bacon was able to develop the porous nickel electrodes needed to convert the energy produced by the reaction between hydrogen and oxygen, into electricity.
The Bacon fuel cell was perfect for powering NASA’s spacecraft: it was lighter and much less bulky than batteries of the time, it was more efficient than 1960’s solar panels, and hydrogen and oxygen were already going to be on board the ship for use as rocket fuel. What’s more, the only waste product from the reaction was water – needed on Apollo 11 for the astronauts to drink.
“Bacon’s fuel cell was a tremendous achievement,” said Professor John Davidson, former Head of Department here at CEB, “The result of a lifetime’s hard work, persistence and ingenuity.”
A prototype of one of the electrodes developed by Bacon was donated to the Whipple Museum of the History of Science in Cambridge and is on proud display.
Watch researchers from our Electrochemical Microengineering Group demonstrate how a fuel cell works for BBC Breakfast and hear from Professor John Davidson, who knew Tom Bacon well:
The science behind the Bacon fuel cell
Unlike heat engines, which have an absolute limit on the efficiency with which they can turn heat into electricity, a fuel cell, can in principal, release all of the free energy of a chemical reaction as electricity. In practice, efficiencies in excess of 70% are attainable. Bacon concentrated on developing a hydrogen-oxygen cell with an alkaline potassium hydroxide electrolyte operating under pressure at about 200°C using non-precious metals. The first problem he had to overcome was establishing a stable interface between the hydrogen and oxygen gases, the liquid electrolyte and the solid electrode; this was finally achieved by means of a bi-porous structure. A small positive differential pressure was maintained between gas and electrolyte. On the gas side of the sintered nickel electrode coarse pores allowed displacement of the liquid electrolyte which was retained by fine pores due to surface tension. This created an interface between all three components within the nickel electrode.
Although these electrodes made it possible to build a fuel cell, rapid deterioration of the oxygen electrode occurred. This problem was finally solved by oxidising the oxygen electrodes in air when coated with lithium hydroxide solution. This treatment lead to the formation of a black semiconducting nickel oxide layer, which was stable under operating conditions. Another vital observation was that by using very high concentrations of the potassium hydroxide electrolyte, the oxygen electrode performance and cell efficiency were greatly improved. This made the Bacon cell superior to alternatives.
Work in the department which had been sponsored by the Electrical Research Association and the Ministry of Fuel and Power culminated in a six-cell, 5-inch diameter power source operating at 200°C and 400psi achieving individual cell EMFs of 0.8V at 230mA/sq cm. Subsequently sponsored by National Research Defence Corporation, the work moved to Marshall of Cambridge where a 40-cell 10-inch diameter hydrogen-oxygen power source producing some 5 kW of power, was built and demonstrated in 1959.
At this time, the proposed Apollo moon probe was designed with hydrogen and oxygen on board for propulsion and life support. The fuel cell was an ideal source of on-board electrical power with the additional advantage that the exhaust water could be used both for drinking by the crew and humidification of the capsule's atmosphere. Because of its high efficiency, the Bacon cell was licensed by the Pratt and Whitney Division of United Aircraft and used in a successful bid to NASA for a $100 million proposal to build the power source for Apollo.
Header image: © NASA
All other images: © University of Cambridge, Department of Chemical Engineering and Biotechnology



