Advances in catalysis and catalytic processes will play a central role in our ability to provide sufficient supplies of renewable energy, in protecting the environment and in developing benign processes for the chemical and pharmaceutical industries. Much of our catalysis research focuses on applying magnetic resonance techniques to catalysts and the reactor environments in which they operate. We also have an emerging interest in the development of THz spectroscopy for application to catalysis. The group works closely with a number of industrial partners, as well as having academic partnerships with the Department of Chemistry and Chemical Engineering at Queen’s University Belfast, and the departments of chemistry at Glasgow and Cardiff.
Particular areas of interest
Understanding the role of carbon in catalytic processes
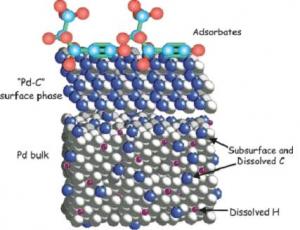 The deposition of carbonaceous deposits (coke) on catalyst surfaces during reaction is most commonly implicated as a major contributor to catalyst deactivation, i.e. loss of catalytic activity. However there is now increasing interest in the role carbon may play in enhancing catalytic activity or selectivity. This may take the form of coke directly catalysing the desired reaction, a phenomenon we have named “active coke”; or through a synergistic interaction between carbon and the catalyst surface. In partnership with the group of Prof. Robert Schlögl at the Fritz-Haber Institute of the MPG in Berlin we are using a number of experimental techniques to characterise the structure of these active and catalytically selective materials (e.g. Teschner et al., J. Catal 242 (2006) 26).
The deposition of carbonaceous deposits (coke) on catalyst surfaces during reaction is most commonly implicated as a major contributor to catalyst deactivation, i.e. loss of catalytic activity. However there is now increasing interest in the role carbon may play in enhancing catalytic activity or selectivity. This may take the form of coke directly catalysing the desired reaction, a phenomenon we have named “active coke”; or through a synergistic interaction between carbon and the catalyst surface. In partnership with the group of Prof. Robert Schlögl at the Fritz-Haber Institute of the MPG in Berlin we are using a number of experimental techniques to characterise the structure of these active and catalytically selective materials (e.g. Teschner et al., J. Catal 242 (2006) 26).
Investigations in Cambridge focus on the use of Tapered Element Oscillating Microbalance (TEOM) techniques to study both carbon deposition and transient adsorption/desorption processes. Related to the concept of “active coke” is the use of carbonaceous materials, in particular carbon nanotubes and carbon nanofibres, directly as catalysts. Such materials are characterised within our group by THz spectroscopy. The application of THz spectroscopy to catalysis opens up the opportunity for us to relate catalytic activity and selectivity to both the electronic and physical characteristics of catalysts (e.g. Parrott et al., J. Phys. Chem. C 113 (2009) 10554).
Green catalysts
An ultimate objective is to design a catalyst that is 100% selective to the product you require. In practice we are a long way from achieving this. Our interest is in using magnetic resonance to understand the working conditions of the real catalyst, e.g. how do reactant and product species interact with the catalyst surface, how do they move within the pore structure of the catalyst? With this knowledge we believe we will be far better placed to design catalysts with enhanced catalytic properties.
Integrated design of catalyst and reactor
However good the catalyst is as a powder, much of the selectivity and activity of the catalyst can be lost when it is operated with the reactor environment in its final form. Much of our work has focused on imaging hydrodynamics (flow-fields) inside fixed-bed reactors. Our current projects also include study of gas-liquid systems and gas solid-fluidized beds.
Sustainable energy production using cyclic gas-solid reactions
Gas-solid reactions in which a porous solid reactant is converted to a solid product and back again provide a means for the sustainable production of energy from coal or biomass. This process often results in a change in the pore size distribution and morphology of the reacting particle. Understanding these changes is critical to developing particles that can be used for a large number of cycles. This approach is of key interest in the capture of CO2 from flue gases and advanced methods of utilising coal, using chemical looping combustion or gasification. Furthermore, these advanced chemical CO2 capture techniques can be used to improve the conversion of industrially critical reactions, e.g. enhancing the water-gas shift reaction by using a catalyst in the presence of a solid sorbent for the CO2.
The Department has a number of industrial collaborators in this field including Johnson Matthey. Dr Andrew York is currently seconded from Johnson Matthey Technology Centre to develop a strategic partnership with the Department.

